MDNA55—A Molecular Trojan Horse
MDNA55 is an Empowered Superkine developed as a therapeutic for recurrent glioblastoma multiforme (rGBM), a uniformly fatal form of brain cancer. By using a highly specific IL-4 Superkine as the vehicle to deliver a potent bacterial toxin to the tumor cells, MDNA55 has the potential to purge bulk tumors and disrupt their supporting networks, while reactivating the immune system to tackle cancer.
Basics of MDNA55
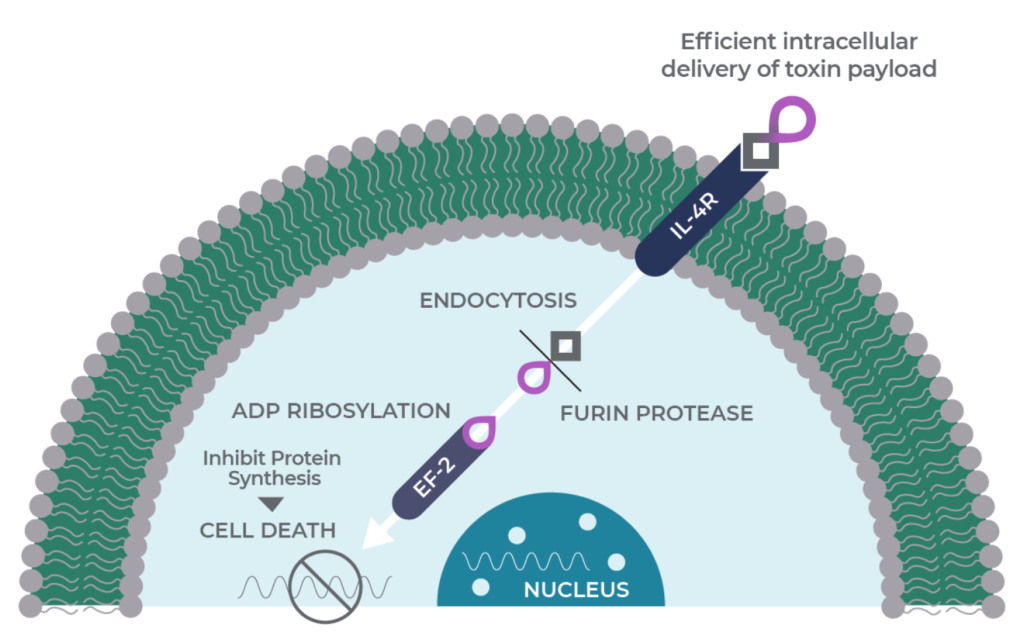
MDNA55 is designed to be a molecular trojan horse. It is a genetic fusion of two molecules: a circularly permuted IL-4 Superkine and the catalytic domain of the pseudomonas exotoxin A.
Genetic fusion allows MDNA55 to harness the selectivity of the Superkine for cancers that overexpress the target IL-4 receptor (IL-4R) and deliver the cell-killing toxin directly into the tumor, its microenvironment and cancer stem cells. Since the IL-4 receptor is not found in a healthy brain and the exotoxin is only active in the cancer cell cytoplasm, this helps ensure that healthy cells are unaffected.
When MDNA55 binds the target IL-4R, it is swallowed inside the tumor cell through a process called endocytosis. Once inside the tumor, proteases cleave the drug and activate the catalytic domain of the exotoxin to begin the process of apoptosis (cell death) involving a protein called elongation factor-2.
Why IL-4R and rGBM
Overexpression of IL-4R is a feature of many cancer types, and has been found to be generally associated with highly aggressive forms of cancer and poor survival outcomes. IL-4R overexpression is also tied to immune system-suppressing mechanisms employed by tumors, which includes the tumor microenvironment, tumor associated macrophages [TAMs], myeloid-derived suppressor cells [MDSCs] and a dominance of Th2 cells over Th1 cells — which favors tumor growth and metastasis.
An analysis of more than 2,000 cancer biopsies, shows that IL-4R is over-expressed in 20 different types of solid and hematological cancers, alongside the MDSCs and TAMs present in the tumor microenvironment. This indicates IL-4R as a potential therapeutic target. What’s more, MDNA55 is uniquely positioned to exploit this receptor and directly disrupt the multiple networks that support a broad range of cancers.
For brain cancers in particular, 75 percent of patients’ tumors overexpress IL-4R. The current standard of care for rGBM does not improve patient survival and remains an area of significant unmet need. Medicenna has opted to focus development of MDNA55 towards rGBM, where it has the potential to provide significant benefit because of its ability to target the bulk tumor and the microenvironment simultaneously.
Clinical Trials
MDNA55 has been studied in four clinical trials in 118 patients with rGBM, in which it has demonstrated compelling indications of superior efficacy to the current standard of care. MDNA55 has been granted Fast Track (FDA) and Orphan Drug Status (FDA and EMA) for the treatment of rGBM.